Implemented by
Implemented by Capital Consulting Corporation
About the “Respirator Fit Evaluation” Challenge
Compete for $350,000 in total prizes and help to improve the health & safety conditions for all respirator users.
Background
The National Institute for Occupational Safety and Health (NIOSH) within the Centers for Disease Control and Prevention (CDC) is the federal organization specifically dedicated to generating new knowledge in the occupational safety and health field and transferring that knowledge into practice for the betterment of workers.
The mission of the National Personal Protective Technology Laboratory (NPPTL) is to advance the state of knowledge and application of personal protective technologies. NPPTL conducts scientific research, leads federal and consensus personal protective technology standards and development, approves respirators, develops guidance and authoritative recommendations, disseminates information, and responds to requests for workplace health hazard evaluations.
Problem Statement
Humans come in many shapes and sizes, as do respirators. This results in wide variability of physical dimensions and features of both people and respirators. The ability of a respirator to form a satisfactory seal or barrier between the wearer and the contaminated environment may be significantly affected by these variabilities. If the respirator-user match (fit) is not checked, an unsatisfactory seal/barrier may unknowingly exist. This could allow excessive leakage of airborne contaminants into the wearer’s breathing zone, despite the user wearing a respirator correctly selected for the application. A fit evaluation is used to address this issue and assess whether a specific type, model and size of respirator can adequately fit a specific individual.[1]
There are issues with the current state of fit evaluation on filtering facepiece respirators (FFRs), especially for those working outside of the U.S. Occupational Safety and Health Administration (OSHA) regulatory oversight.
- Research and anecdotal information reveal that health workers at doctors’ offices, nursing homes, dental facilities, and home health settings may not conduct initial or annual fit evaluation on a consistent basis due to lack of resources and fit evaluation procedures being impractical to implement widely across an organization
- From a global pandemic, to wildfire smoke, and air pollution in urban areas; now more than ever the general public is wearing tight fitting respirators without the benefit of a fit evaluation to know if the respirator selected is properly providing the intended level of protection.
- Under serious outbreak conditions, current approaches to quantitative and qualitative fit evaluations are constrained and lack the ability to efficiently scale.
These problems present an opportunity for innovators to design a respirator fit evaluation solution delivering users real-time information on a filtering facepiece respirators (FFRs) fit.
[1] INTRODUCTION TO RESPIRATOR FIT EVALUATION, https://tsi.com/getmedia/25c8d870-4544-4ae4-8f24-a13a27819577/Introduction-to-RFT-(ITI-070)-US?ext=.pdf
How to Enter
In order to enter the Challenge, team’s should follow these three steps.
- Register your team. Each team is required to identify a Team Leader who registers the official Team on the external Challenge site.
- Attend the informational webinar on February 2, 2023. Register and attend the virtual informational webinar to gain additional information and ask clarifying questions to the Challenge team.
- Submit a Phase 1 Concept Paper by May 12, 2023. Phase 1 submission materials are due on the external Challenge site by 11:59 PM EDT on May 12, 2023. View the Phase 1 Concept Paper submission details in the Submission Requirements section of this document.
Challenge Goal
The NIOSH Respirator Fit Evaluation Challenge will crowdsource novel technologies and innovative approaches that deliver immediate evaluation and feedback to end users about the fit of filtering facepiece respirators (FFRs) during use. Solutions should consider how to provide immediate feedback to a diverse group of end users, including faces within the NIOSH anthropometric grid[2] as well as faces of under-represented populations or those falling on the edges of the NIOSH anthropometric grid are encouraged.
Some possible solutions for this challenge may include:
- Innovations for determining fit on initially putting on the respirator
- Innovations for determining fit continuously while being worn
- Qualitative or quantitative fit evaluation solutions
Please note: Solutions that may meet the criteria in ANSI standard Z88.10 for fit evaluation methods, and therefore may be considered by OSHA, and others, as a valid method are welcome. However, it is not a requirement of the challenge to meet the ANSI standard Z88.10.
[2]NIOSH Anthropometric Data and ISO Digital Headforms; https://www.cdc.gov/niosh/data/datasets/rd-10130-2020-0/default.html
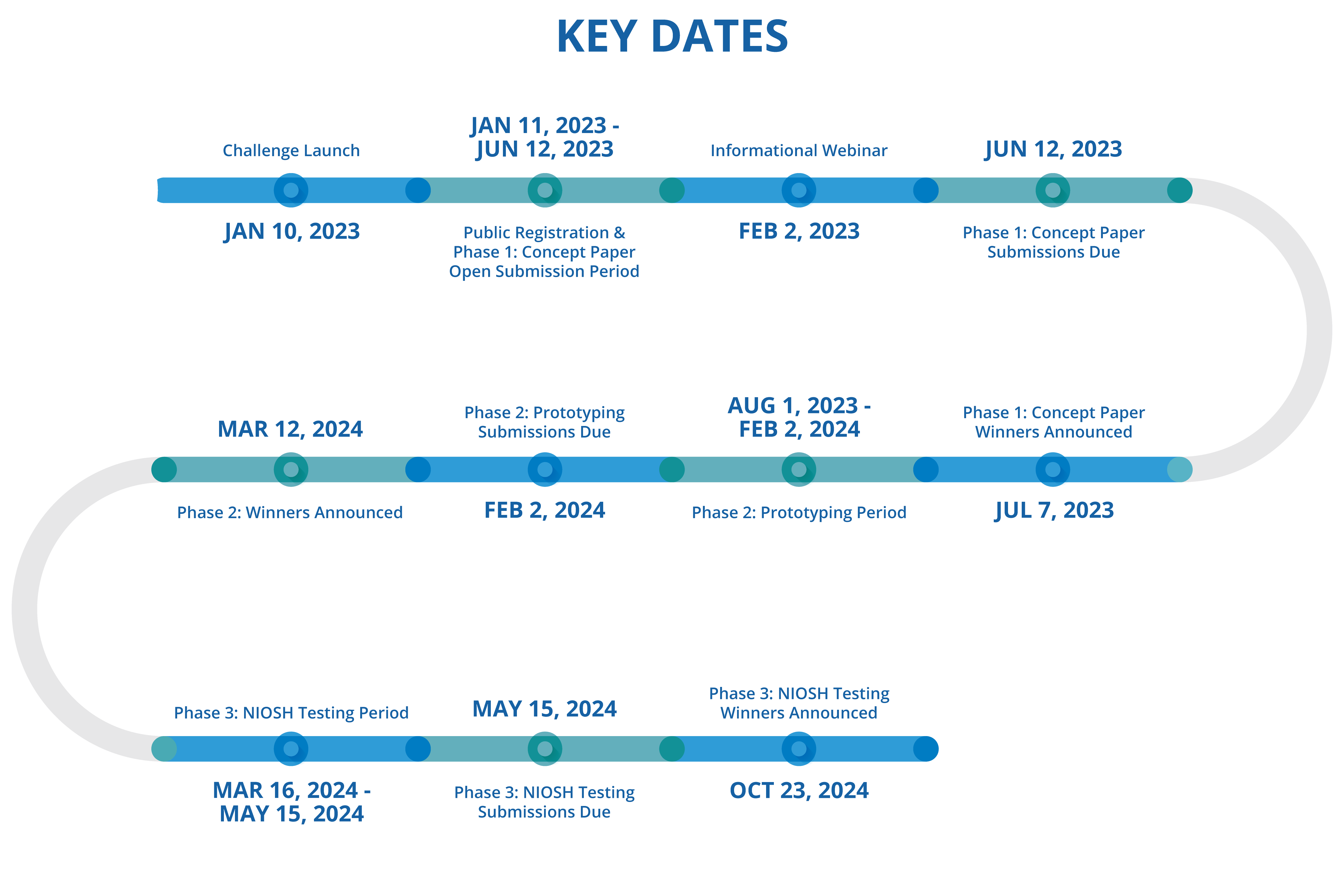
Key Dates
| Activity | Key Dates |
|---|---|
| Challenge Launch | 01/10/2023 |
| Public Registration & Phase 1 Concept: Open Submission Period | 01/11/2023 – 05/01/2023 |
| Informational Webinar | 02/02/2023 |
| Phase 1 Concept Paper: Submissions Due | 05/12/2023 |
| Phase 1 Concept Paper Winners Announced | 07/07/2023 |
| Phase 2: Prototyping Period | 08/01/2023 – 02/02/2024 |
| Phase 2: Prototyping Submissions Due | 02/02/2024 |
| Phase 2 Winners Announced | 03/12/2024 |
| Phase 3: NIOSH Evaluation Period | 03/16/2024 – 04/05/2024 |
| Phase 3: NIOSH Evaluation Submissions Due | 05/15/2024 |
| Phase 3: NIOSH Evaluation Winners Announced | 10/23/2024 |
Prizes and Phase Overview
Prize Program Structure
The Challenge will be implemented as a three-phase, $350,000 competition collecting concept papers in Phase 1, building and evaluating prototypes in Phase 2, and sharing end solutions with NIOSH in Phase 3 for evaluation. By the end of the competition, solutions should aim to be market-ready with user-friendly fit evaluation solutions for end users of FFRs.

| Phase | Phase Description | Submission | # of Awards | Total Prize Purse |
|---|---|---|---|---|
| Phase 1: Concept Paper | This is the open submission period.
Competitors will submit a description of their solution. Winners of this phase will receive a monetary award and may receive an invitation to participate in Phase 2. |
10-page Concept Paper | Up to 20
$5,000 per award |
$100,000 |
| Phase 2: Prototyping | Selected winning concepts will build a prototype based on their submission. Monetary awards will be structured to incentivize competitors during this phase. | Evaluation Report
Video demonstration of Solution |
Up to 10
$10,000 per award |
$100,000 |
| Phase 3: Demonstration & NIOSH Evaluation | Selected winning submissions from Phase 2 will build pre-production prototypes for NIOSH evaluation. Prototypes may be evaluated using qualitative and quantitative test methods. Winners of this phase will receive a monetary award. | Functional Prototype delivered for Evaluation | 3
1st place: $75,000 2nd place: $50,000 3rd place: $25,000 |
$150,000 |
Submission Requirements
Phase 1: Concept Paper
Phase 1 Contestants will submit a 10-page concept paper. Proposals must be no longer than 10 pages, with up to 2 optional pages for letters of commitment or support.
Phase 1: Concept Paper (10 pages)
| Submission Component | Component Description | Recommended Page Length |
|---|---|---|
| Executive Summary | Describe the overall team, solution, and data collection approach. | 1 |
| Team Description and Organizational Qualifications | Describe your team and the primary qualifications of your team and organization as it pertains to Fit Evaluation. | 1 |
| Fit Solution and Evaluation Approach | Describe your proposed solution and the overall fit evaluation approach. | 3 |
| Prototype Sketch | Develop a sketch, work flow, or visual representation of the fit solution. | 1 |
| Ability to address the Challenge Goal | Describe how your solution addresses the problem statement in an innovative and impactful manner. | 1 |
| Data collection and measurement approach | Describe your method for collecting results and immediately delivering results to those performing fit evaluations. | 2 |
| Plans for Prototyping & Evaluation | Describe your plan for prototyping and evaluation if you were to be selected for Phase 2 & Phase 3. | 1 |
Phase 2: Prototyping
Phase 2 contestants will develop verifiable prototypes of their fit evaluation solutions and conduct small-scale evaluations. The findings from their evaluation and prototype development efforts must be documented in a Phase 2 Prototype Demonstration Report and visually captured in a 3-5 minute product demonstration video. The Phase 2 Evaluation Report and Product Demonstration Video will be submitted for Phase 2 judging and evaluation.
Phase 2: Prototype Demonstration Report (5-10 pages or presentation slides).
MS Word or MS PPT are both acceptable deliverable formats.
| Submission Component | Component Description | Recommended Page Length |
|---|---|---|
| Executive Summary | Describe the overall team, solution, and data collection approach. | 1 |
| Development Process | Describe how the solution was prototyped including key materials and production techniques. | 1 |
| Evaluation Methodology | Describe how the solution was evaluated, defining the specific indicators or metrics used to evaluate fit. | 2-3 |
| Key Findings | Describe the key results of conducting the small-scale evaluation. | 2 |
| Areas of Improvement | Describe how the team will optimize or enhance the end solution based on user feedback and/or product limitations. | 2 |
Phase 2: Prototype Demonstration Video (3-5 minutes)
Videos should be shared via an accessible link such as YouTube or Vimeo. If teams are interested in preserving the privacy of the videos, they can configure the link visibility to be ‘Private’ so that only judges with the correct link can access the video.
| Submission Component | Component Description | Recommended Page Length |
|---|---|---|
| Product Demonstration Video Link | Communicate how the fit evaluation solution works as demonstrated on a user, how the fit evaluation solution is set-up and administered, and how results should be interpreted. | No more than 5 minutes |
Phase 3: NIOSH Evaluation
To compete for the Phase 3 NIOSH Fit Evaluation prizes, innovators will be required to submit to NIOSH staff a sufficient number of pre-production prototype devices, or their equivalent, along with any ancillary devices, sufficient instructions/procedures for device operation, results interpretation, and any controls for evaluation to NIOSH staff.
| Submission Component | Component Description | Recommended Page Length |
|---|---|---|
| Product Instructional Manual | Document describing how NIOSH should set-up and implement the solution for evaluation purposes. | 3-5 pages |
| Physical Product(s) Shipped to NIOSH location | The prototype and all required components* required to implement the fit evaluation solution. | n/a |
*If software is a required component, teams must also ship a laptop with the software pre-installed to streamline the process for the NIOSH evaluation team.
Evaluation Criteria
Phase 1: Concept Paper Judging Criteria
The determination of the winners will be made by a panel of judges, as determined by NIOSH, based on the following evaluation criteria.
| Evaluation Criteria | Guiding Criteria Question | Criteria Weighting |
|---|---|---|
| Team Qualifications | Does the proposed team have the experience and capabilities to develop a functional prototype? | 10% |
| Technical Feasibility | Can the described solution be feasibly developed within a 6-month timeframe? | 30% |
| Ease of Use | Is the proposed solution usable for both care providers and filtering facepiece respirator (FFR) users? | 20% |
| Fit Evaluation Potential | Can the proposed solution effectively evaluate a user’s FFR fit? | 20% |
| Impact Potential | To what extent can the proposed solution improve upon the status quo of fit evaluation and/or end user knowledge of NIOSH-Approved® respirators that fit properly? | 20% |
Phase 2: Prototyping Judging Criteria
The determination of the winners will be made by a panel of judges, as determined by NIOSH, based on the following evaluation criteria.
| Evaluation Criteria | Guiding Criteria Question | Criteria Weighting |
|---|---|---|
| Technical Results | To what extent has the prototype delivered the intended results to end users? | 30% |
| Ease of Use | Is the proposed solution usable for both test providers and respirator users as demonstrated in the product demonstration video? | 20% |
| Execution of Proposed Project Plan | To what extent has the team delivered on their proposed plan described in Phase 1? | 20% |
| Impact Potential | To what extent can the proposed solution improve upon the status quo of fit evaluation and/or positively impact the overall market? | 20% |
| Market Readiness | To what extent does the solution have the potential to go to market? | 10% |
Phase 3: NIOSH Evaluation Judging Criteria
To enter the NIOSH Evaluation Phase, teams will be required to ship a sufficient number of pre-production final prototypes devices and all required components to a defined NIOSH facility. After physically receiving the end solutions and all required instructional components, NIOSH will independently evaluate the solution’s performance and provide a report of the results to a judge panel who will make the final decision on Phase 3 prize awards.
Based on the end products received, possible tests may include:
- Verification of submitted results
- Advanced headform fit evaluation
- Bench testing for stated functionality
- Additional tests based on the unique characteristics of an end solution
Intellectual Property (IP) Treatment
Intellectual Property (IP) Treatment
Innovator grants to the Federal government an irrevocable, paid-up, royalty-free, non-exclusive, worldwide license to reproduce, publish, post, link to, share, and display publicly the submission on the web or elsewhere, and a non-exclusive, non-transferable, irrevocable, paid-up license to practice, or have practiced for or on its behalf, the solution throughout the world. Each Innovator will retain all other intellectual property rights in their submissions, as applicable. To participate in the Challenge, each Innovator must warrant that there are no legal obstacles to providing the above-referenced nonexclusive licenses of the Innovator’s rights to the Federal government.
To receive a prize for their submission, Innovators must agree to grant the Federal government a non-exclusive license right to the inventive concept or inventions and patents in all Intellectual Property demonstrated by the winning/award submissions. Full intellectual property rights also remain with the licensor.
Eligibility Requirements
Eligibility Requirements
- The Prize is open to anyone age 18 or older participating as an individual or as a team.
- Individual competitors and teams may originate from any country, as long as United States federal sanctions do not prohibit participation.
- If you are a federal employee, your participation in this Challenge is prohibited.
- Submissions must originate from either the U.S. or a designated country (see definition of designated country at https://www.acquisition.gov/far/part-25#FAR_25_003), OR have been substantially transformed in the U.S. or designated country prior to prototype delivery pursuant to FAR 25.403(c).
- Submissions must be made in English. All Challenge-related communication will be in English. You are required to ensure that all releases or transfers of technical data to non-U.S. persons comply with International Traffic in Arms Regulations (ITAR) 22 C.F.R. §§ 120.1 to 130.17. Submissions from outside individuals and non-expert teams are encouraged. All applications will go through a process of due diligence; any application found to be misrepresentative, plagiarized, or sharing an idea that is not their own will be automatically disqualified.
- All ineligible applicants will be automatically removed from the competition with no recourse or reimbursement.
- No purchase or payment of any kind is necessary to enter or win the competition.
- By participating in this Challenge, each participant (whether an individual, group of individuals, or entity) agrees to indemnify the Federal Government against third-party claims for damages arising from or related to Challenge activities.
- Participants shall not use the NIOSH, CDC, or HHS names, logos or official seals in their submissions and must not claim endorsement from those entities.
- Participants agree that CDC may disqualify the submission if, in CDC’s judgment, the program is inconsistent with CDC’s public health mission, may be ineffective or harmful, or any other reason deemed necessary.
- Record Retention and FOIA: All materials submitted to HHS as part of a submission become HHS records and cannot be returned. Any confidential commercial information contained in a submission should be designated in accordance with 45 C.F.R. § 5.41. Participants will be notified of Freedom of Information Act requests for their submissions in accordance with 45 C.F.R. § 5.42.
Implemented by
Implemented by Capital Consulting Corporation